

Messenger RNA (mRNA) Vaccines
Moderna’s mRNA-1273 vaccine, The COVE StudyTM
The mRNA-1273 vaccine is being developed to prevent COVID-19. The purpose of this study is to test Moderna’s vaccine candidate to see if it can prevent illness if people are exposed to the SARS-CoV-2 virus in their everyday lives. The mRNA-1273 vaccine is not made from the SARS-CoV-2 virus. It is made from messenger ribonucleic acid (mRNA), a genetic code that tells cells how to make protein, which helps the body’s immune system make antibodies to fight the virus. The vaccine cannot cause infection or make someone sick with COVID-19.
Visit ClinicalTrials.gov - NCT04470427 for additional details about the Moderna COVID-19 vaccine clinical study.
Pfizer & BioNTech’s mRNA Vaccine Study
BNT162b2 is an investigational mRNA vaccine being developed by Pfizer and BioNTech to help prevent COVID-19, the disease caused by the SARS-CoV-2 virus. The purpose of the study is to test how well the investigational vaccine works at preventing COVID-19 disease and evaluate whether it is safe for adults and adolescents. BNT162b2 contains a small part of the genetic code for the SARS-CoV-2 spike protein. BNT162b2 does not contain any live virus. It cannot give you SARS-CoV-2 infection, nor will you get COVID-19.
Visit ClinicalTrials.gov - NCT04368728 for additional details about the Pfizer and BioNTech COVID-19 vaccine clinical study.
About Messenger RNA Vaccines
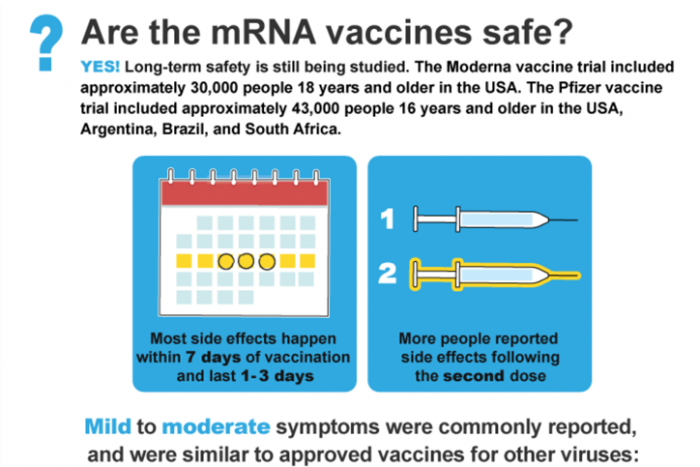
mRNA – Are they safe?
Long-term safety is still being studied, but YES they are safe! Most side effects happen within 7 days of vaccination and last 1-3 days. More people reported side effects following the second dose. Read more about mRNA vaccines safety and side effects.

mRNA – How do they work?
mRNA vaccines give your body the information needed to defend against a virus. Read an overview of how mRNA vaccines work and the importance of receiving 2 doses.

mRNA – What are the benefits?
After receiving 2 doses, mRNA vaccines can reduce the risk of being hospitalized with severe COVID-19 disease and dying as a result of severe COVID-19 symptoms. Read more about the benefits and information on priority groups included in the vaccine trials, underscoring safety and efficacy for these groups.

mRNA – What do we still hope to find out?
There are many reasons why continued research for other COVID-19 vaccines is needed. Until we know more, we should continue wearing masks. Read more about what we hope to learn with more research.
Content last reviewed on May 5, 2021
